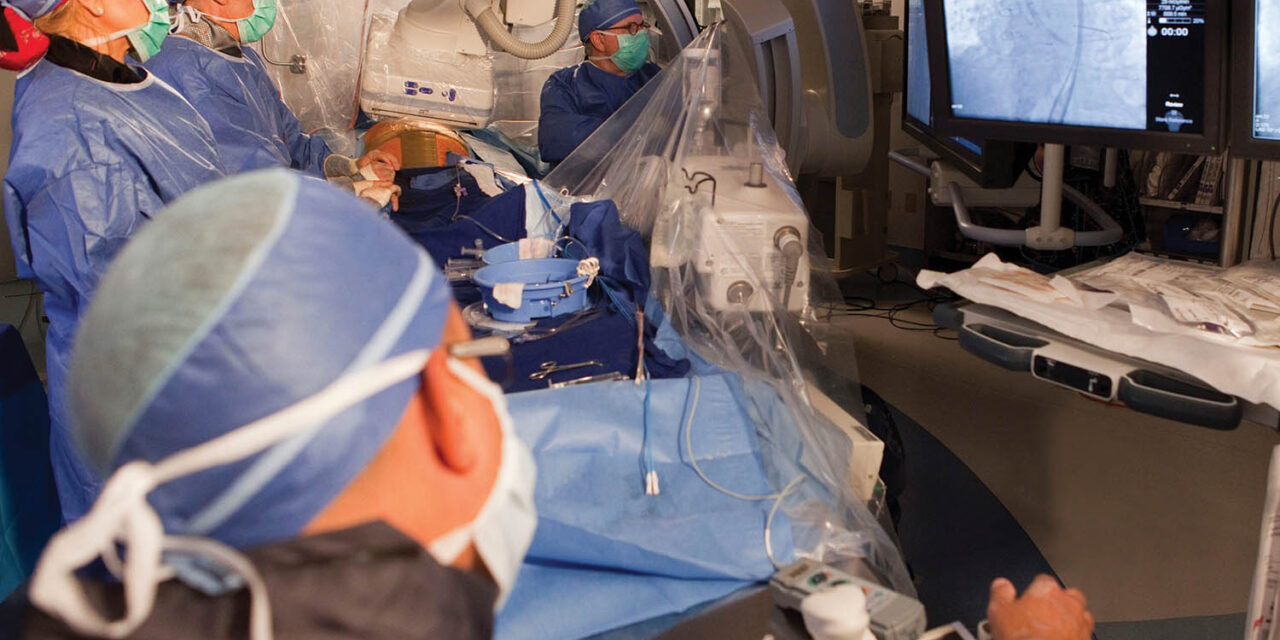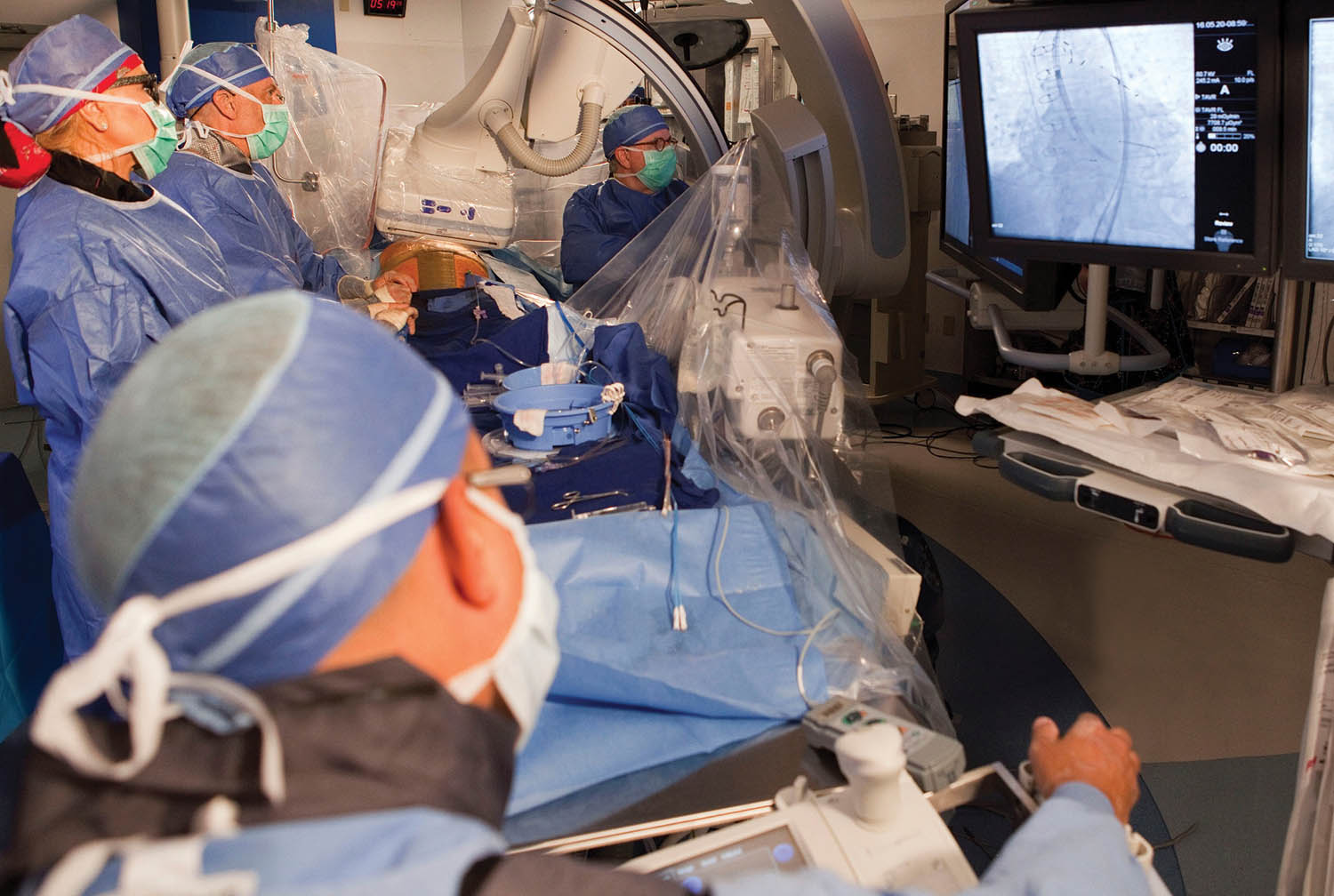
Transcatheter aortic valve replacement is a minimally invasive procedure in which a new valve is deployed into the heart via a catheter inserted through a vein to reopen the diseased aortic valve (photo courtesy of Abrazo Health).
More than two million patients live with moderate aortic stenosis in the U.S. And now, the first randomized clinical study evaluating the Evolut™ transcatheter aortic valve replacement (TAVR) platform in patients with moderate, symptomatic aortic stenosis will come to Phoenix.
Aortic stenosis occurs when the heart’s aortic valve narrows due to calcium buildup. It is a progressive disease, meaning it gets worse over time, and can be debilitating, costly and deadly. Abrazo Arizona Heart Hospital is one of the select sites worldwide to participate in the EXPAND II Pivotal Trial. The Evolut TAVR platform currently is approved for the treatment of symptomatic severe aortic stenosis patients across all risk categories (extreme, high, intermediate, and low) in the U.S.
Abrazo says that TAVR is a minimally invasive procedure to reopen the diseased aortic valve by inserting a prosthetic valve. The trial will enroll up to 650 patients globally to study the safety and efficacy of the procedure in patients with moderate, symptomatic aortic stenosis. The data from the study may be used to support future regulatory submissions to expand the current indications for the Evolut TAVR platform.
For more information on the study design, visit www.clinicaltrials.gov (NCT05149755).